OUR BUSINESS
MANAGEMENT
ACHIEVEMENTS
45
FDA IND
APPROVALS
1,000
PATENTS
FILED
$100ms
START UP
FUNDS RAISED
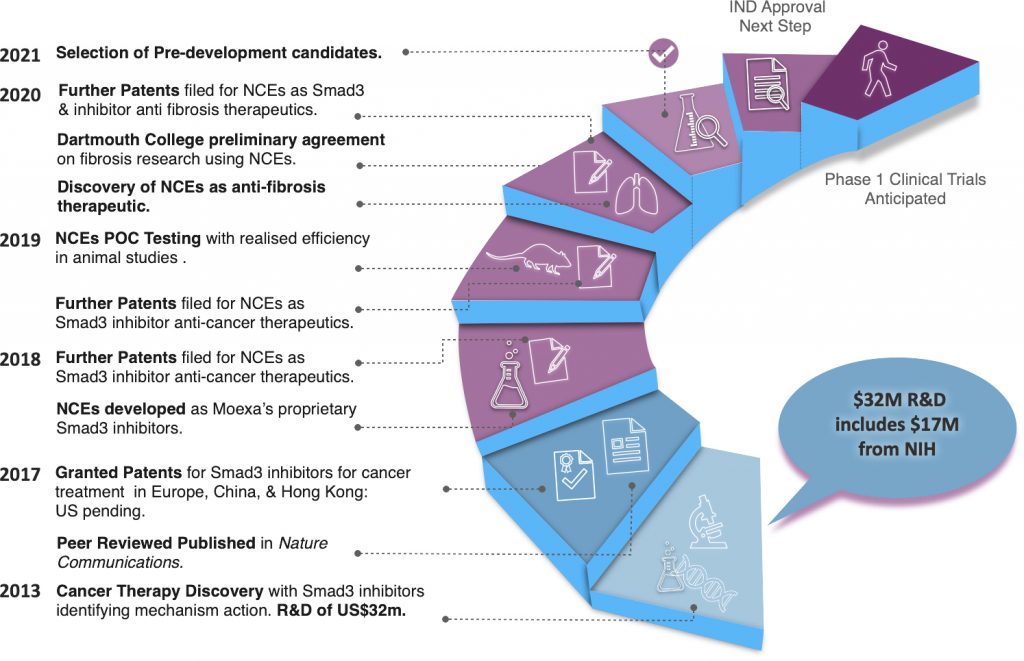
We are a multi national that has developed novel therapeutics focusing on TGF-β/Smad3 signalling applications and solutions. Our business development has advanced to refining pre-IND protocols for FDA filings in anticipation of clinical trails. We are actively seeking collaboration, investment and partnership opportunities that will accelerate getting our products to market.
Our original R&D was completed with $32m in funding, including $17m in grants from NIH. Our technology’s peer review has been published in Nature Communications. We have since created proprietary and successfully POC tested NCEs that have application as immuno-oncology cancer therapeutics.
Our NCE also demonstrate therapeutic efficacy as anti-fibrotics addressing interaction between coronavirus nucleocapsid proteins following COVID infections. We have planned future product development by arranging research collaboration with the Geisel School of Medicine at Dartmouth College in Hanover, N.H.
We have a robust patent library protecting coverage for mechanism-of-action for many cancers, specified anti-fibrotic applications, and promoting our NCE for further drug development. Our patent portfolio includes granted or pending patents in the USA, Europe, China, Canada, Australia, India, and Hong Kong.
